Please be aware that ResMed have recently sent out an Urgent Field Safety Notice regarding several of their masks. We are writing to you to make you aware of the information contained in their letter and the instructions you should follow if you are impacted by this issue.
ResMed has provided us with important information regarding their masks with magnets which may interact with some implants or certain medical devices. These masks are used to deliver air to a patient from a positive airway pressure (PAP) device.
Magnets are used in some ResMed masks that provide a simple and easy way for patients to attach and detach the headgear to the mask frame as detailed in the picture below.
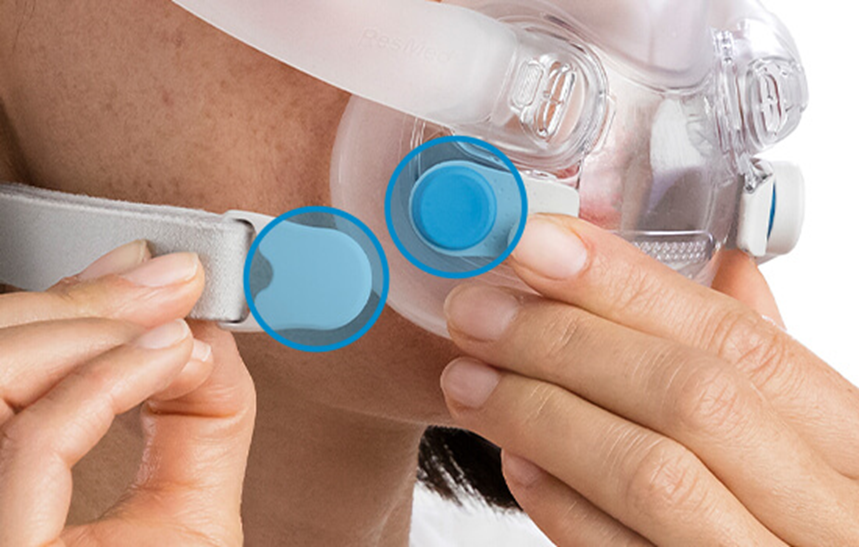
Resmed is updating its contraindications and warnings to further inform patients and healthcare professionals on the safe use of ResMed masks with magnets.
Does this apply to me?
If you do not use a mask with magnets, the updated contraindications and warnings do not apply to you.
The updated contraindications for ResMed masks with magnets only apply if you, or anyone in close physical contact with you while using the mask (e.g., bed partner), has a certain implant or medical device that is identified below.
If you use a ResMed mask with magnets, you should also be aware of the updated warning noted below.
Updated contraindications
Masks with magnetic components are contraindicated for use by patients where they, or anyone in close physical contact while using the mask, have the following:
Updated warning
Keep the mask magnets at a safe distance of at least 6 inches (150mm) away from implants or medical devices that may be adversely affected by magnetic interference. This warning applies to you or anyone in close physical contact with your mask. The magnets are in the frame and lower headgear clips, with a magnetic field strength of up to 400mT. When worn, they connect to secure the mask but may inadvertently detach while asleep.
Implants/medical devices, including those listed within contraindications, may be adversely affected if they change function under external magnetic fields or contain ferromagnetic materials that attract/repel to magnetic fields (some metallic implants, e.g., contact lenses with metal, dental implants, metallic cranial plates, screws, burr hole covers, and bone substitute devices). Consult your physician and manufacturer of your implant/other medical device for information on the potential adverse effects of magnetic fields.
Which ResMed masks are affected:



All of the above are full face masks

All of the above are Nasal Masks
What to do next:
The mask is safe to carry on using however, what to do if you suspect you are in one of these groups:
Then
Patients should stop using the affected mask if the implant/medical device is contraindicated against the mask magnets.
Contact Vivisol, your service provider who has a good supply of masks and you should swap out in the event of you being in one of the impacted groups above.
Patients should consult their physician immediately to determine if another mask can be used for their therapy.
Please contact Vivisol Customer Service on: 0800 121 4012
Patients should properly dispose of the mask that has magnets after an alternative is obtained.
Registered Office Address:
Dolby Medical Home
Respiratory Care Ltd
North Suite, Lomond Court,
Castle Business Park, Stirling,
FK9 4TU
Registered in Scotland
No. 063902
Telephone:
Home oxygen enquiries: 0800 833 531
Respiratory support: 0800 121 4012
Gatwick Office Address
Vivisol UK
Palladian, Manor Court,
Manor Royal,
Crawley,
RH10 9PY
Telephone:
Home oxygen enquiries: 0800 917 9840
Respiratory support: 0800 121 4012
Follow Us
Useful links
News sign-up
By subscribing you will always be update with the latest news from us.
© Copyright 2025 Vivisol. All Rights Reserved.
To find out more about The Vivisol Group and The SOL Group click on the links here: The VIVISOL Group | The SOL Group